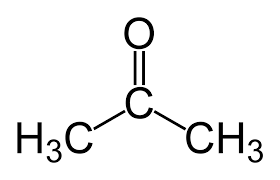
DESCRIPTION
Industrial Uses
- Adhesives and sealant chemicals
- Agricultural chemicals (non-pesticidal)
- Intermediates
- Ion exchange agents
- Laboratory chemicals
- Processing aids, not otherwise listed
- Processing aids, specific to petroleum production
- Solvents (for cleaning or degreasing)
- Solvents (which become part of product formulation or mixture)
- Viscosity adjustors
- Adhesives and Sealants
- Fabric, Textile, and Leather Products not covered elsewhere
- Paints and Coatings
- Personal Care Products
- Plastic and Rubber Products not covered elsewhere
- Toys, Playground, and Sporting Equipment
Manufacturing
Nearly 90% of acetone production is via cumene. In this process, acetone is coproduced with phenol. The main production process involves the reaction of propylene and benzene in the presence of phosphoric acid-based or zeolite catalysts. Cumene is oxidized in the liquid phase to cumene hydroperoxide. It is then cleaved in the presence of sulfuric acid to phenol and acetone. Approximately 0.62 tons of acetone is produced with each ton of phenol.





Reviews
There are no reviews yet.